Mb: Single chain with a similar folding as a Hb subunit; serve as O2 storage protein in muscles (i.e., diving mammals)
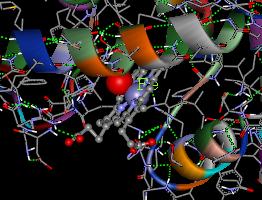
Below is a picture of the heme center of Hb when it binds an oxygen (represented as a red sphere).
I believe by now you have set up the CD accompanied
with the book on your computer. You might notice that there is a
program called WebLab ViewerPro that came with the CD. It is an excellent
program for viewing molecules (see the two images below created with this
program). The one with the book is an old version (3.12), and the latest
version (3.7) costs $279! If you want to view the structures that come
with the CD, including myoglobin, you may just run the program and open
the file you want to view. The program can open many different structure
files, including the files from the Protein Data Bank with an extension
pdb. There is a folder called "Molecule" on the CD, from which you can
find a good collection of many molecules. Since those files are pdb
files, you need to change the "files of type" to "Brookhaven pdb files"
in order to open the pdb files. Myoglobin structure has the file
name "Myoglobi.pdb".
In case you feel like to see other protein or
nucleic acid structures, the Protein
Data Bank is the place to visit! Currently, there are nearly
16,000 structures in the bank!
Hb: Made up of four heme
groups surrounding a globin group (2a and 2b
chains), forming a tetrahedral structure; found in the red blood cells
Mb: Single chain with a
similar folding as a Hb subunit; serve as O2 storage protein
in muscles (i.e., diving mammals)
Below is a picture of the heme center of Hb when it binds an oxygen
(represented as a red sphere).

Heme (structure on P. 610) contains iron and gives intense colors, depending on what it binds.
Bright red when binds O2
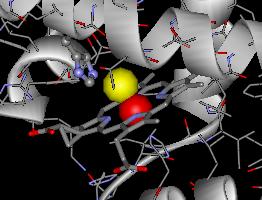
Red/pink when binds CO (~200 times better
than O2)
Dark red/purple when binds H2O
Brown when Fe(II) is oxidized to Fe(III)
—Cannot bind O2 anymore!
Other forms/mutants of hemoglobin in human:
A (normal), B, C, D, E, F (fetal, more efficient in O2
binding), H, and S (sickle
cell Hb, b chain glutamate at
the 6th position is mutated into a valine.)
Other O2 carriers:
Hemocyanin in molluscs and Arthropods
(hemo--blood; cyan--blue; and -in about proteins)
Cu-containing (2Cu+ in each protein molecule)
Changes from colorless to blue upon O2 binding
Hemerythrin in some sea worms (heme
means blood; erythro means red; -in is "protein")
Fe-containing (2Fe2+ in each protein subunit)
Changes from nearly colorless to burgundy upon O2
binding
There are many good Websites about hemoglobin and myoglobin, listed
below:
http://web.indstate.edu/thcme/mwking/hemoglobin-myoglobin.html
http://sickle.bwh.harvard.edu/hemoglobin.html
or you may want to search for more in the Web!
About oxygen O2
Is oxygen harmful?
When oxygen oxidizes glucose and other "energy sources" in the food,
it changes into water, i.e., oxygen is reduced. This overall reaction looks
just fine!
However, during the reduction of oxygen, a couple of intermediates
are formed, which are superoxide radical (O2–•) and
peroxide (O22–) with one and two electron reduction
of an oxygen molecule, respectively. Thus, an oxygen molecule requires
a total of 4 electrons to completely reduced to water.
The two intermediates are very cytotoxic, and are considered some of
the factors in causing cancers, arthritis, and other disorders.
Superoxide radicals can be catalyzed into peroxide and water by the enzyme superoxide dismutase (SOD) in our body. (A 3D structure, the active site, and some physical studies of this enzyme can be found at the website, click here.) Mutations on this enzyme have recently been characterized to be associated with amyotrophic lateral sclerosis (ALS) or Lou Gehrig's Disease. Here is a website (http://www.melisa.org/nl2099.html) which has a brief description about SOD and ALS. Rich sources about ALS can be found on the Web.
Peroxide can be produce free radicals when react with Fe(II) and Cu(I)
ions and their complexes. The free radicals produced then can cause
damages on proteins and nucleic acids. Hydrogen peroxide (H2O2)
in our body can be converted into oxygen gas and water by the enzyme catalase
according to the following equation.
2H2O2 ---> O2 + 2H2O
The oxygen generated here is what the bubbles you see when you put
2% hydrogen peroxide solution on minor cut.