such as O (e.g., ethanol, H3C—CH2—OH)
N (e.g., amines R–NH2)
S (e.g., cysteine)
halogens (e.g., chloroform CHCl3 and Freons, CFCs)

Electron configuration of carbon atom: 1s2 2s22p2, i.e., 4 VE
Electron/Lewis dot structure: ???
A carbon atom can form single, double, or triple bonds.
Carbon atoms can form covalent bonds
with hydrogen atoms,
e.g., methane (CH4)
and propane (C3H8) etc.
e.g., Carbon-hydrogen
bond: C + 4•H ®
CH4
with other carbons to build
long chains,
e.g., ethane (H3C—CH3),
ethylene (H2C=CH2), etc.
Writing and drawing formulas and structures,
e.g., butane, C4H10
1) CH3–CH2–CH2–CH3;
2) CH3CH2CH2CH3;
3) CH3(CH2)2CH3
4) C—C—C—C;
5) Draw all atoms.
with other elements,
such as O (e.g.,
ethanol, H3C—CH2—OH)
N (e.g., amines
R–NH2)
S (e.g., cysteine)
halogens (e.g.,
chloroform CHCl3 and Freons, CFCs)

Hydrocarbons—Compounds contains only H and C.
Long chains or rings can be built by C–C linkages to give compounds

Naming hydrocarbons (P. 293)
1C methane
methyl: —CH3
2C ethane
ethyl: —CH2CH3
3C propane
4C butane
5C pentane
6C hexane
7C heptane
8C octane
9C nonane
10C decane
Structural isomers—Compounds
with the same formula but different molecular structures
Example: C5H12

Primary (1º, bound to another C), second (2º, 2 other C’s), tertiary (3º, 3 other C’s), and quaternary (4º, 4 other C’s) carbons, see above example.
Multiple bonds
Alkenes contain C=C double bonds
e.g., ethene
(ethylene) C2H4, propene (propylene)
C3H6
Geometric isomers—due to the
rigidity without free rotation along the C=C bond (whereas single bond
can have free rotation)
Examples: cis
and trans 2-butene/pentene, etc.
Alkynes—containing triple bonds
Reactions on >C=C<
Hydrogenation:
adding H atoms to "saturate" the double bond for the preparation of margarine.
>C=C< + H2 (+catalyst) ®
>CH–CH<
Hydration
with water: formation of alcohol
>C=C< + H2O (+catalyst) ®
>CH–COH<
Halogenation
with HX or X2
>C=C< + HCl (+catalyst) ®
>CH–CCl<
>C=C< + Br2 (+catalyst) ®
>CBr–CBr<
Polymerization
n H2C=CH2 (+catalyst) ®
–(CH–CH)n– (polyethylene, PE)
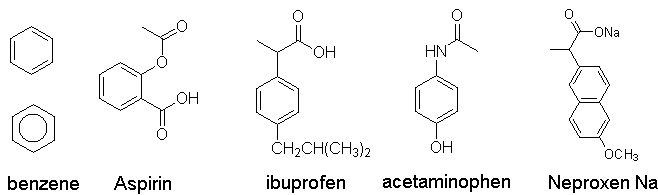
Aromatic compounds: a fundamental
structural motif of drugs
e.g., benzene (C6H6)
Sources of hydrocarbons: Petroleum, natural gas, and coal
Petroleum refining—Partial distillation of crude oil into fractions of HCs according to boiling point
Cracking—a controlled process by which catalysts are used to break down or rearrange natural HCs into more useful molecules, such as high-octane gasoline.
Properties of HCs and their substituents:
hydrophobicity—essential
property in proteins
to be functionalized—producing
other useful compounds
completely oxidized
to produce H2O and CO2—the greenhouse gas
Alcohols and phenols— Compounds
containing R–OH group
H–O–H (replacement
of one H by R) ®
R–OH (alcohol)
R–OH (replacement
of H by R’) ®
R–O–R’ (ether)
The –OH group can be involved in H-bonding.
Nomenclature
alkane ®
alkanol
methane
® methanol
ethane ®
ethanol
Formation??
Reactions
Dehydration to form
alkenes (the reverse reaction of alkene hydration), or ether
CH3CH2CH2–OH
(H2SO4, 180º) ®
CH3CH=CH2
2 R–OH (H2SO4,
140º) ® R–O–R
+ H2O
Oxidation to form aldehydes (1º alcohol, with the –OH attached to a 1º carbon) or ketones (2º alcohol). 3º alcohols are not oxidized into other compounds under mild conditions.
Esterification: forming esters
with carboxylic acids
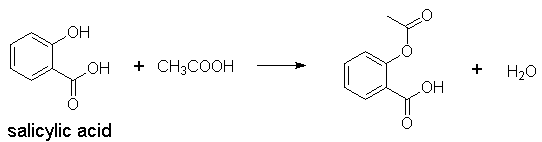
Ethers
solvent, anesthetics, etc.
Formed via dehydration of alcohols
Thiols: Distinct odors!!
e.g., from skunk,
natural gas additive (ethanethiol which we can sense at 0.1 ppb).
Aldehydes and Ketones
in nature: flavors of almond, cinnamon, vanilla, mint, and butter; hormones;
sugars; etc. cf. PP. 406, 407.
Both contain a
>C=O group
Nomenclature
Change the ending -e of the alkanes
into -al for aldehydes and into -one for ketones, e.g., propane ®
propanal or propanone (P. 410)
Formation??
Reactions
Oxidation of aldehydes gives
carboxylic acids, whereas ketones are not oxidized under mild conditions.
RCHO (oxidation)
® RCOOH
Reductions of aldehydes and
ketones give 1º and 2º alcohols, respectively.
In human body,
the carbonyl group >C=O can be reduced by nicotinamide adenine dinucleotide
(NADH) in association with enzymes, e.g., reduction of pyruvic acid. See
P. 421.
Amines
"Derivatives" of ammonia with –R group(s):
1 R, 1º amines; 2 R’s, 2º amines; and 3 R’s, 3º amines.
All amino acids have 1º amino group except proline, which has a 2º
amino group (P. 589).
Naming: "Alkyl" + amine, e.g., methylamine, butylamine, etc.
Amines can be in cyclic structures,
as found in the amino acid histidine, in nucleic acids, in vitamine B,
opium alkaloids, nicotine, caffeine, cocaine, etc.
|
Physical properties: Controlled by the bond polarity and H-bonding |
 |
Naming: Change the ending -e
in alkanes to –oic acid.
e.g., butane ®
butanoic acid
Physical properties of carboxylic acids are controlled by their bond polarity and H-bonding, cf. PP. 438 and 439.
Carboxylic acids in nature: formic acid, acetic acid, citric acid, lactic acid, pyruvic acid, amino acids, etc.
Soaps are sodium of potassium salts of long-chain carboxylic acids (or fatty acids). The long chains are hydrophobic that interact with grease-like stuff, and the –COO–Na+ (or K+) part is hydrophilic that interact with water and makes soap "soluble" in water (which actually forms micelles). What would happen to soap if the water is "hard"? (See P. 445)
Reactions
1) Form esters with alcohols,
e.g., formation of aspirin, formation of heroin from morphine via simple
acetylation, etc.
R–COOH + R’OH ®
R–CO–OH + H2O
Thus, the reverse
reaction is the hydrolysis of esters.
Reaction of a dicarboxylic
acid with a diol can produce a polymer, a polyester.
HOOC–R–COOH + HO–R’–OH ®
HOOC–R–COO–R’–OH + H2O
Add another acid ®
another diol …®
® ®
® a polyester!!
2) Form amides with amines,
e.g., formation of peptide bonds (linking amino group of one amino acid
with the carboxyl group of another amino acid)
R–COOH + R’NH2
® R–CO–NHR + H2O
Thus, the reverse
reaction is the hydrolysis of amide bonds, including the hydrolysis of
a peptide bond.
Reaction of a dicarboxylic acid with
a diamine can produce a polymer, e.g., nylone-66 is formed by linking 1,6-hexandioic
acid with 1,6-hexanediamine:
HOOC(CH2)4COOH
+ H2N(CH2)6NH2 ®
–(CO(CH2)4–CONH–(CH2)6NH)n
How about formation of proteins? See P. 595.
Both the >C=O group and the NH group can be involved in H-bonding.
Phosphoric acid can also form esters, phosphoesters, which are the backbone of nucleic acids!!