Proton NMR Studies of Co(II) Complexes of Bacitracin Analogs
—Insight into Structure-Activity Relationship
Jon D. Epperson and Li-June Ming*
Department of Chemistry and
Institute for Biomolecular Science
University of South Florida
Tampa, Florida 33620-5250
Abstract
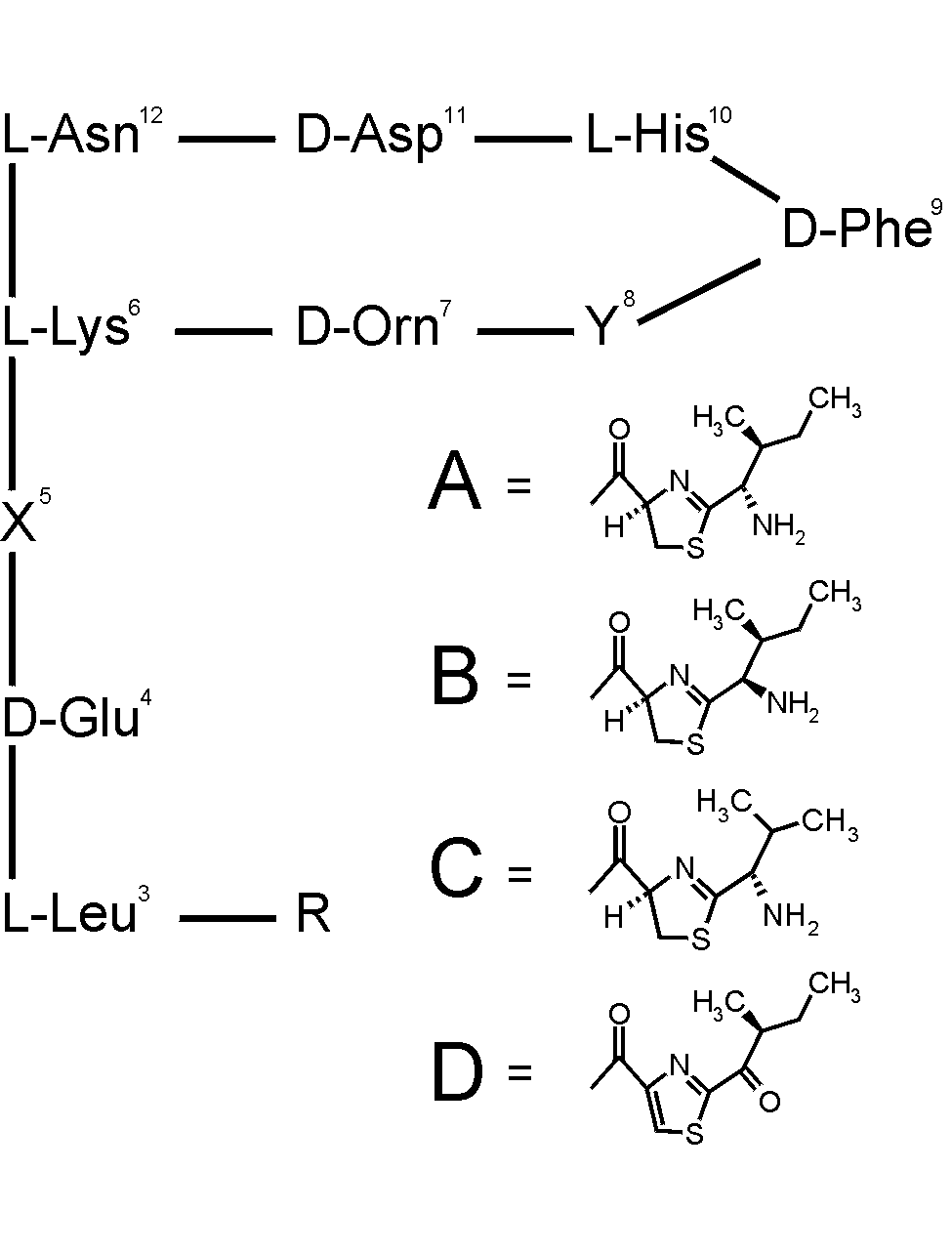
Bacitracin is one of the most commercially and medically important
antibiotics that has been produced in large quantity (see structure below).
It is a metal dependent antibiotic produced by Bacillus subtilis
and Bacillus licheniformis with a potent antimicrobial activity
directed primarily against Gram positive bacteria. This antibiotic
requires a divalent metal ion such as Zn(II) for its antimicrobial activity,
and has been reported to also bind several transition metal ions, including
Co(II), Ni(II), and Cu(II). Despite the wide use of this antibiotic,
the structure and its relationship with activity of metal-bacitracin complexes
have never been determined and fully understood. The Co(II) complex
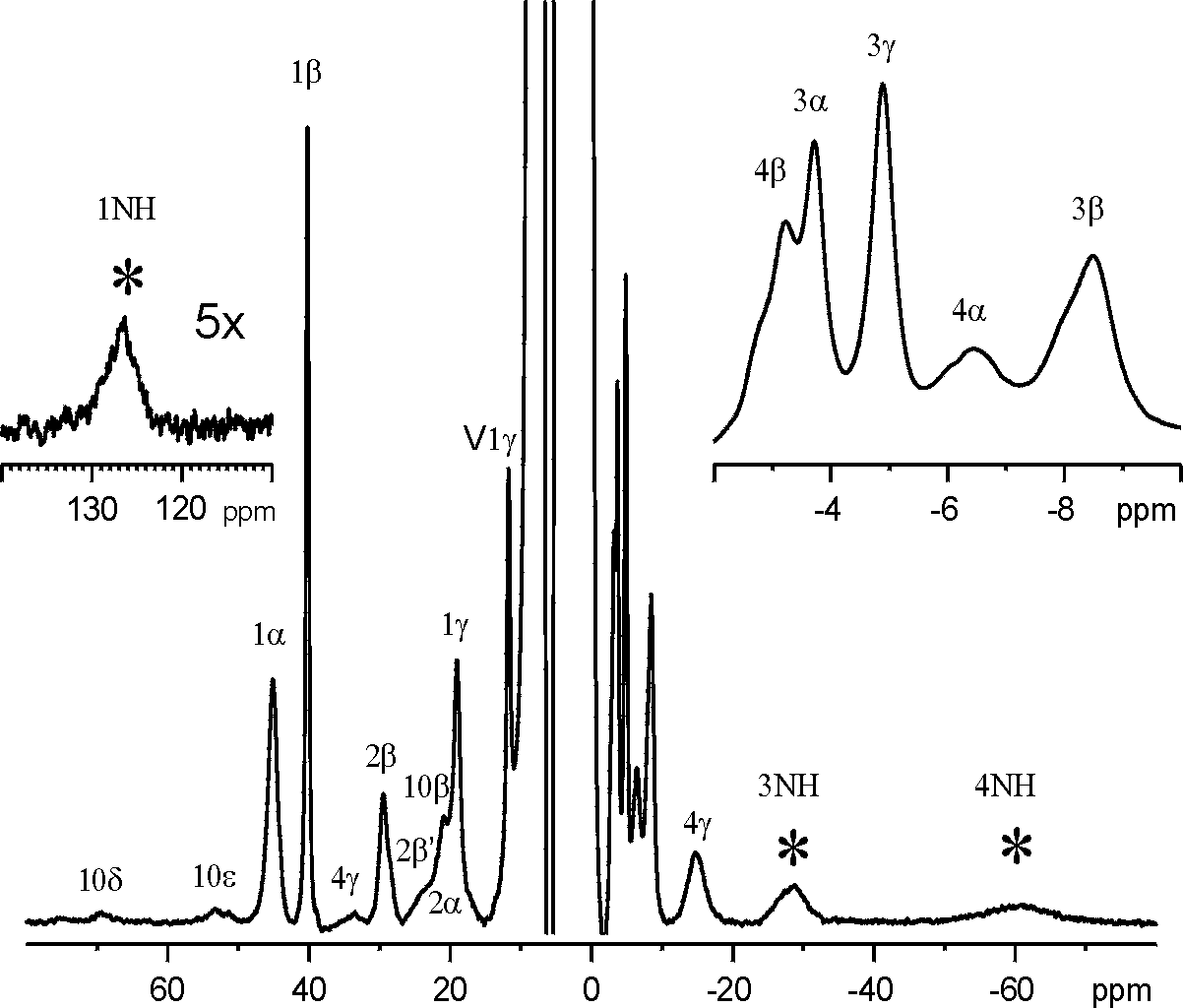
of this antibiotic exhibits several hyperfine-shifted 1H
NMR signals in a large spectral window (~150 ppm) due to both contact and
dipolar shift mechanisms (see spectrum below). These shifted features
have been conclusively assigned by means of 1D and 2D NMR techniques.
The NMR studies show that this drug binds to Co(II) via the His-10 imadazole
ring, the thiazoline ring nitrogen, and the Glu-4 carboxylate to form a
labile complex. The free amine of Ile-1 does not bind the metal ion
based on the assignment of the NMR signals. Several different analogs
of bacitracin have been prepared, and their Co(II) binding properties have
been studied by the use of NMR techniques. The results indicate that
the antimicrobial activity of these analogs correlates directly to their
metal binding capability and the metal binding mode. The binding
of Glu-4 to the metal seems to play a critical role in the antibiotic activity
of bacitracin since it is found not to bind the metal in the analogs with
low antibiotic activities, including bacitracins A2 and F and desamido-bacitracin
A1.

(Back to Current Publications)