Jon D. Epperson, Li-June Ming*, Barry D. Woosley, Gregory R. Baker,
and George
R. Newkome*
Department of Chemistry, the Institute for Biomolecular Science,
and the Center for Molecular Design & Recognition, University of South
Florida, Tampa, FL 33620
* To whom correspondence should be addressed.
L.-J. Ming (813) 974-2220, ming@chuma.cas.usf.edu
G.R. Newkome Telefax: Int. code – (813) 974-4962, www.dendrimers.com
Abstract
Cobalt(II) has been utilized as a paramagnetic 1H
NMR probe to investigate the internal cavities of dendrimers possessing
specifically located binding sites. T1 values
of the hyperfine-shifted signals were used to calculate metal-proton distances
to build a molecular model of the internal structure of the dendrimers.
The presence of sizeable cavities within the dendrimers was observed including
a loosely packed conformation for the 2,6-diaminopyridine moiety to bind
to potential guest molecules.

Introduction
The unique supramolecular chemistry associated with
dendritic macromolecules has raised great interest in the structural and
chemical characteristics of these polymers.1
For example, the incorporation of specific recognition sites located within
the dendritic structure has permitted chemical processes like H-bonding
and metal ion complexation to occur at these loci. Indeed, many dendrimers
have been suggested to loosely mimic the structure and function of globular
proteins owing to their large size, spherical shape, and specific host-guest
chemistry.1b Unlike proteins, however,
dendrimers have not been successfully analyzed by X-ray crystallography
due to their fractal nature that interrupts the long range molecular ordering
necessary for crystal packing and X-ray diffraction.2
Dendrimers also exhibit broad overlapping 1H NMR features which further
hinders the analysis of their conformations in solution.
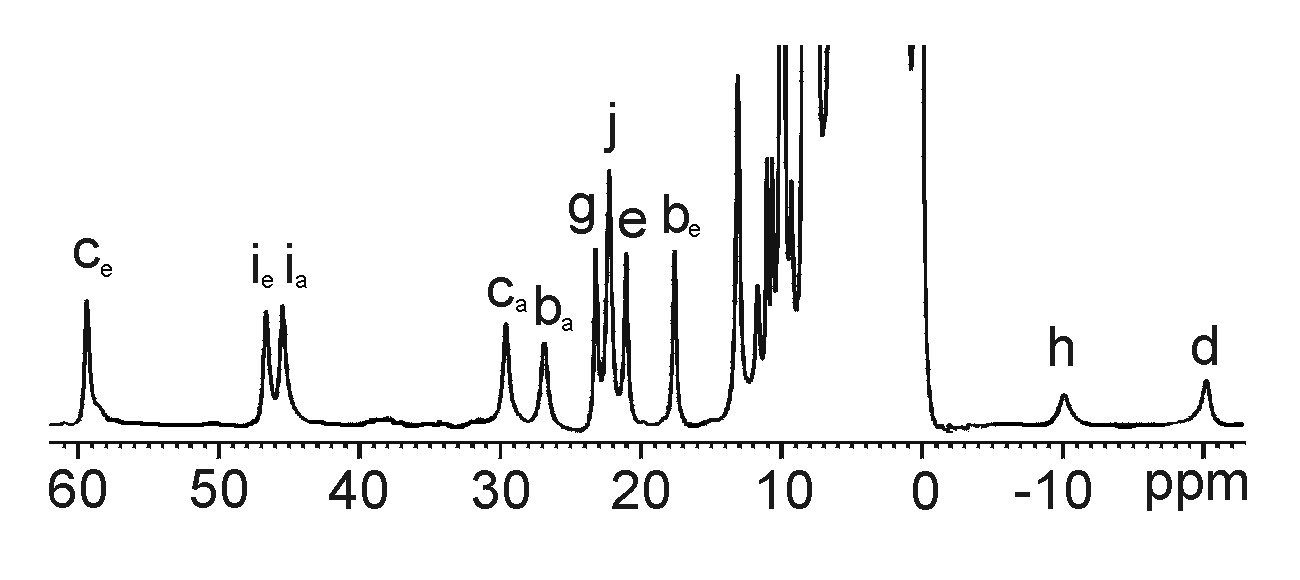
Paramagnetic metal ions with short electron relaxation
times (<10–11 s), especially Co(II),
have been extensively used as intrinsic and external NMR probes for the
analysis of the active sites of metalloproteins.3
Protons close to these paramagnetic metal centers can exhibit isotropically
shifted (or hyperfine-shifted) 1H NMR signals
with short relaxation times T1 and T2.3
These signals can be significantly shifted outside the crowded diamagnetic
region at 0-10 ppm which facilitates their detection and assignment.
Presumably, isotropically shifted 1H NMR
signals for paramagnetic metal-bound dendrimers, if detected, can be especially
useful for the characterization of these macromolecules that exhibit poorly
resolved overlapping 1H NMR features.
For example, a few dendrimers which contain intrinsic paramagnetic Fe-S
cores have been shown to display some well-resolved hyperfine-shifted 1H
NMR signals with small chemical shifts.4
For dendrimers lacking any intrinsic paramagnetic centers, simple paramagnetic
metal ions such as Co(II) can be utilized as external probes for detailed
investigation of the environment about metal recognition site(s), if present.
The availability of dendrimers possessing four potential metal binding
2,6-diamidopyridine sites5 affords a simple
system to further evaluate this principle for the first time. The
diamidopyridine functional groups in these dendrimers have previously been
demonstrated to be involved in H-bonding with guest molecules.5
Thus, these dendrimers may serve as prototypes for functional dendrimers.
We herein describe the use of the paramagnetic metal ion Co(II) as an external
NMR probe for detailed investigation of dendrimer structure.
References
(1) For examples, see: a) Narayanan,V.V.; Newkome, G. N. Topics
Curr. Chem. 1998, 197, 19-77. b) Zimmerman, S.
C. Chem. Rev. 1997, 97, 1681-1712. c) Bosman,
A. W.; Janssen, H. M.; Meijer, E. W. Chem. Rev. 1999, 99,
ASAP Article. d) Archut, A.; Vögtle, F. Chem. Soc.
Rev. 1998, 27, 233-240.
(2) Ottaviani, M. F.; Bossmann, S.; Turro, N. J.; Tomalia, D. A. J.
Am. Chem. Soc. 1994, 116, 661-671.
(3) a) Bertini, I.; Luchinat, C. NMR of Paramagnetic Molecules
in Biological Systems, Benjamin/Cumming: Menlo Park, CA, 1986.
b) Berliner, L. J.; Reuben, J. Eds.; NMR of Paramagnetic Molecules,
Plenum: New York, 1993. c) La Mar, G. N. Ed. Nuclear Magnetic
Resonance of Paramagnetic Macromolecules, NATO-ASI, Kluwer: Dordrecht,
Netherlands, 1995. d) Bertini, I.; Luchinat, C. Coord. Chem. Rev.
1996, 150. e) Ming, L.-J. In Physical Methods in
Bioinorganic Chemistry, Spectroscopy and Magnetism, Que, L. Jr., Ed.,
University Science Books: CA 1999.
(4) a) Gorman, C. B.; Hager, M. W.; Parkhurst, B. L.; Smith,
J. C. Macromolecules 1998, 31, 815-822. b) Gorman,
C. B.; Parkhurst, B. L.; Chen, K. Y.; Su, W. Y. J. Am. Chem. Soc.
1997, 119, 1141-1142.
(5) a) Newkome, G. R.; Woosley, B. D.; He, E.; Moorefield, C.
N.; Guther, R.; Baker, G. H.; Escamilla, G. H.; Merrill, J.; Luftmann,
H. Chem. Commun. 1996, 2737-2738. b) Breinlinger, E.;
Niemz, A.; Rotello, V. M. J. Am Chem. Soc. 1995, 117,
5379-5380.
(Back to Current Publications)