Xiangdong Wei and Li-June Ming*
Department of Chemistry and
Institute for Biomolecular Science
University of South Florida
Tampa, Florida 33620-5250
USA
Abstract
The antibiotic streptonigrin (SN) produced by Streptomyces
flocculus is a metal-dependent potent antitumor agent. Optical
and 1H NMR techniques have been applied
to the study of a few metal complexes (Co2+,
Fe2+, and Yb3+)
of this antitumor antibiotic. The hyperfine-shifted 1H
NMR signals of these paramagnetic complexes are fully assigned by means
of NMR relaxation, EXSY, and COSY techniques. These studies reveal
that SN binds transition metal and lanthanide ions and form stable metal-drug
complexes, with the metal located at the quinolinequinone-pycolinate sites.
This configuration requires a ~180º twist of the C2—C2’ bond in the
crystal structure of the drug. The hyperfine-shifted 1H
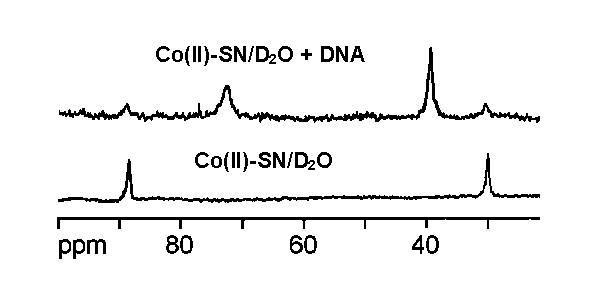
NMR signals of the Co2+-SN complex are
significantly changed upon addition of calf thymus DNA or poly[dA-dT],
indicating direct binding of Co2+-SN complex with DNA.

(Read more about streptonigrin.)
(Read an introduction about metal complexes of the antitumor antibiotics
anthracyclines.)