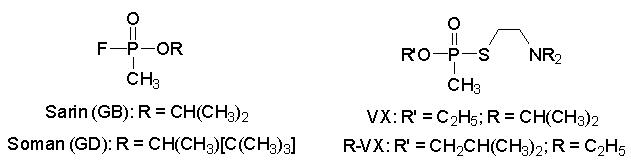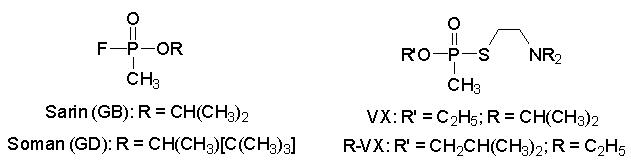
Although proteins with different functions can have nearly identical folding and/or structural motifs, proteins possessing a single active site that exhibit different functions are not commonly seen. We have recently observed that sAP exhibits an unexpected opportunistic activity, i.e., phosphodiesterase activity. The detailed studies of the two different types of hydrolysis by sAP may lead to a better understanding of and unifying the mechanisms of dinuclear hydrolytic reactions in chemical and biological systems. From a chemical viewpoint, the protein moieties in metalloproteins can be considered as “macromolecular ligands” which in many cases bind preferably only certain metal ions. Thus, the idea of using “protein ligands” for the preparation of different “macromolecular metal complexes” to serve as “natural chemical models” may point new directions for chemical modeling studies.
Degradation of Nerve Agents:
The United States ratified the Chemical Weapons
Convention Treaty in 1997, which bans the use of chemical agents in wars
and possession of the agents, and also prohibits the production and stockpiling
of these agents. Thousands of tons of stockpiles of several chemical warfare
agents are thus awaiting for being destroyed, which thus creates great
safety and environmental concerns. The nerve agents (see structures
below), represented by the VX agent ethyl S-2-(diisopropylamino)ethyl methylphosphonothiolate,
the R-VX agent isobutyl S-2-(diethyl)ethyl methylphosphonothiolate, and
the “G agents” 2-propyl methylphosphonofluoridate (sarin) and 3,3-dimethyl-2-butyl
methylphosphonofluoridate (soman), are potent acetylcholinesterase inhibitors
which inhibit the enzyme via the formation of the indefinitely stable phosphoester
with the active-site nucleophilic Ser. Degradation of these nerve
agents can be achieved by hydrolysis of the P–F and the P–OR bonds and
by oxidative cleavage of the P–SR bond (to yield R–SO3–),
which also represents a valuable practice of hydrolytic and oxidative chemistry.