PARAMAGNETIC LANTHANIDE(III) IONS AS NMR PROBES FOR BIOMOLECULAR
STRUCTURE AND FUNCTION
LI-JUNE MING
Department of Chemistry
and Institute for Biomolecular Science
University of South Florida
Tampa, Florida 33620-5250
[Abstract]
1. Introduction
Paramagnetic transition metal ions have been used successfully as spectroscopic probes for the study of metal binding sites in many metalloproteins [1,2]. When a paramagnetic metal ion with fast electronic relaxation rates is used as a probe, 1H NMR spectroscopy becomes a valuable tool for such study via the detection and assignment of isotropically shifted signals outside of the normal 0-13 ppm region attributable to protons close to the metal ion [2]. Information about the coordinated ligands and the interactions with substrates analogues and the medium can thus be obtained. For example: A coordinated His can be identified via the detection of the isotropically shifted solvent exchangeable imidazole NH signal and its correlation with a ring CH signal, and a coordinated carboxylate-containing residue can also be identified by showing the isotropically shifted (CgH2)-CbH2-CaH spin patterns, which can be differentiated from the spectral features of Cys and Met showing different chemical shifts and relaxation times. The metal ions Fe2+/3+, Co2+, and Ni2+ have been used extensively as probes for structural and functional studies of many metalloenzymes by means of NMR techniques. Particularly, Co2+-substituted derivatives of Zn enzymes can always show high activity, thus can serve as good models for better understanding of the structure and mechanism of the native enzymes [2]. The contributions in this volume by Lawrence Que (on nonheme and non-FeS Fe proteins) and by J. M. Moratal and Antonio Donaire (on Co2+- and Ni2+-substituted proteins) provide a general view on the use of NMR techniques for the study of the coordinated ligands in several paramagnetic metalloproteins.
In addition to the widely used paramagnetic transition metal ion NMR probes, paramagnetic lanthanide(III) ions (Ln3+) with short electronic relaxation times (~10–13 s) have long been used as shift reagents to simplify the assignment of complicated 1H NMR spectra [3] and for the study of biomolecules.[4,5] However, the use of these Ln3+ (as well as other paramagnetic transition metal ions) as NMR probes for the study of the structure and mechanism of proteins has been hampered due to the lack of appropriate techniques for conclusive signal assignment. The use of 2D NMR techniques for the study of paramagnetic metal sites in metalloproteins has been impeded in the past several years owing to the fast nuclear relaxation rates of the isotropically shifted signals, although these techniques have been extensively used for structural studies of diamagnetic proteins [6]. In the past three years, such techniques have been applied successfully in our [7] and other laboratories [8] for the study of the metal sites in several paramagnetic metalloproteins.
Dipolar shift is the predominant isotropic shift mechanism in paramagnetic Ln3+ complexes, as opposed to the presence of large contribution of contact shift mechanism in paramagnetic transition metal complexes (although dipolar shift is also an important shift mechanism in Fe-heme systems as discussed in several other chapters in this volume, such as the ones by Gerd La Mar and James Satterlee), because the valence f orbitals are highly shielded and cannot participate in direct chemical bonding [3]. The dipolar shift of a nucleus depends on the relative position of the nucleus with respect to the metal as shown below [2,3]:
where r is the nucleus-metal distance, q is the geometric angle of the r vector and the Z axis, and W is the angle of the X axis and the projection of the r vector on the XY plane. The configuration of the metal site is obtainable when the principal components of the magnetic susceptibility tensor (c's) are known, although which are not always available in practice [3]. Moreover, the isotropically shifted signals which are buried in the diamagnetic envelope cannot be easily resolved and assigned; and the signals with similar isotropic shifts and relaxation times may also render signal assignment difficult solely based on eq 1. Although proton-metal distances can be estimated by the Solomon equation and Curie relaxation [2], the structure of the metal site cannot be uniquely defined unless the spatial and bond correlations of the nuclei with respect to each other can be precisely determined. One advantage of utilizing paramagnetic Ln3+ ions over transition metal ions as probes is that their causing large dipolar shift of nearby nuclei allows the study of the whole metal environment, but not restricted to only the coordinated ligands as in most cases of the latter.
Proton NOE techniques have been used successfully for the assignment of isotropically shifted resonances in the past several years [8]. The determination of NOE between a pair of nuclei (i and j) in a paramagnetic species can give the internuclear distance (rij) as shown in eq 2 [8,9],
where sij
= – (h/2p)2g4tc/10rij6
is the cross relaxation with g the gyromagnetic
ratio and tc
the rotational correlation time, ri
is the intrinsic relaxation rate of i, and t is saturation
duration time. A NOESY [9,10] experiment can also be described by
a slight modification of eq 2 to include rj
[11]. Coherence transfer NMR techniques have also been useful for
signal assignment in paramagnetic species when cross signals in COSY (as
functions of sin(pJabt1)exp(–t1/T2)
with Jab the scalar coupling constant,
T2 the spin-spin relaxation time,
and t1 the evolution time for the
second dimension) [10] due to scalarly coupled nuclei could be observed.
These techniques have recently been applied for the study of paramagnetic
metalloproteins [7,8] (which are also discussed in several contributions
of this volume such as the theory and technique-oriented ones by Bertini
and by Luchinat), although the observation of relaxation-based coherence
transfer in a COSY experiment becomes possible when Curie relaxation is
present (to a less extent for a small S = 1/2 metalloprotein at
elevated temperatures) [12]. A precise assignment of the isotropically
shifted features, along with the geometric-dependent dipolar shift, can
provide detailed structural information about the paramagnetic metal-binding
environment in metalloproteins. In this contribution, the application
of 1D and 2D NMR techniques for structural and mechanistic studies of several
metallo-biomolecular systems in our laboratory using paramagnetic Ln3+
as probes will be summarized and discussed.
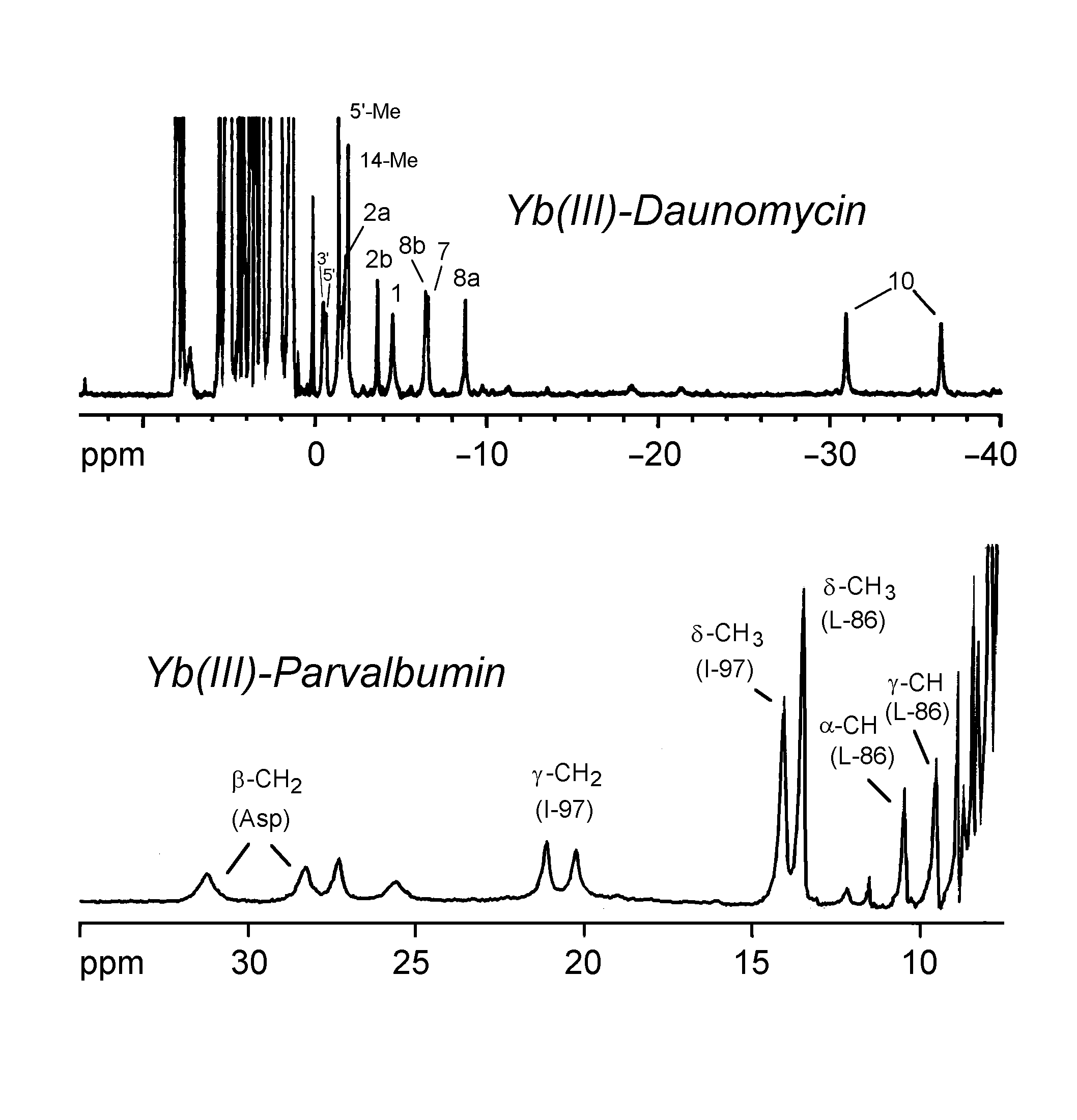
The spectra below show the use of Yb(III) as an NMR probe in the study of daunomycin and parvalbumin
References
[1] Williams, R. J. P. (1978) Enzyme action:
Views derived from metalloenzyme studies, Chem. Britain 14, 25.
(b) Hughes, M. N. (1981) The Inorganic Chemistry of Biological Processes;
2nd Ed.; Wiley, New York.
[2] (a) Bertini, I. and Luchinat, C. (1986) NMR
of Paramagnetic Molecules in Biological Systems; Benjamin/Cumming,
Menlo Park, CA. (b) Berliner, L. J. and Reuben, J., eds. (1993) NMR
of Paramagnetic Molecules; Plenum, New York.
[3] (a) Morrill, T. C., Ed. (1986) Lanthanide
Shift Reagents in Stereochemical Analysis; VCH, NY. (b) La Mar,
G. N., Horrocks, W. DeW., Jr., and Holm, R. H. eds, (1973) NMR of Paramagnetic
Molecules, Chapters 12 & 13; Academic, NY.
[4] Lenkinski, R. E. (1984) Lanthanide complexes
of peptides and proteins, Biol. Magn. Reson. 6, 23-71.
[5] Lee, L. and Sykes, B. D. (1980) High resolution
NMR, Adv. Inorg. Biochem. 2, 183-210.
[6] Wüthrich, K. (1986) NMR of Proteins
and Nucleic Acids; Wiley, NY.
[7] (a) Holz, R. C., Que, L., Jr., and Ming, L.-J.
(1992) NOESY Studies on the Fe(III)Co(II) Active Site of the Purple
Acid Phosphatase Uteroferrin, J. Am. Chem. Soc. 114, 4434-4436.
(b) Bertini, I., Luchinat, C., Ming, L.-J., Piccioli, M., Sola, M., and
Valentine, J. S. (1992) Two-dimensional 1H NMR studies of the paramagnetic
metalloenzyme copper-nickel superoxide dismutase, Inorg. Chem. 31,
4433-4435. (c) Ming, L.-J., Lynch, J. B., Holz, R. C.,
and Que, L., Jr. (1994) One- and two-dimensional 1H
NMR studies of the active Site of iron(II) superoxide dismutase from Escherichia
coli, Inorg. Chem. 33, 83-87. (d) Ming, L.-J. (1993) Two-dimensional
1H NMR studies of Ca(II)-binding site in
proteins using paramagnetic lanthanides(III) as probes and Yb(III)-substituted
bovine a-lactalbumin as an example, Magn.
Reson. Chem. 31, S104-S109. (e) Ming, L.-J. (1993) Two-Dimensional
1H NMR Study of Paramagnetic Lanthanide(III)-Substituted
Ca(II) Proteins, J. Inorg. Biochem. 51, 99.
[8] For general reviews, see (a) La Mar, G.
N. and de Ropp, J. S. (1993) NMR Methodology for Paramagnetic Proteins,
In Berliner, L. J.; Reuben, J., Eds.; NMR of Paramagnetic Molecules;
Plenum, New York.. (b) Bertini, I., Turano, P., Vila, A. J. (1994)
Nuclear Magnetic Resonance of Paramagnetic Metalloproteins, Chem. Rev.
93, 2833-2932.
[9] (a) Noggle, J. H.and Schirmer, R. E. (1971)
The Nuclear Overhauser Effect; Academic, NY. (b) Neuhaus,
D. and Williamson, M. P. (1989) The Nuclear Overhauser Effect in Structural
and Conformational Analysis; VCH, NY.
[10] Ernst, R. R., Bodenhausen, G., and Wokaun,
A. (1987) Principles of Nuclear Magnetic Resonance in One and Two Dimensions;
Oxford.
[11] Sette, M., de Ropp, J. S., Hernandez, G., and
La Mar, G. N. (1993) Determination of interproton distances from NOESY
spectra in the active site of paramagnetic metalloproteins: Cyanide-inhibited
horseradish peroxidase, J. Am. Chem. Soc. 115, 5237-5245.
[12] Bertini, I, Luchinat, C., and Tarchi, D. (1993)
Are true scalar proton-proton connectivities ever measured in COSY spectra
of paramagnetic macromolecules? Chem. Phys. Lett. 203, 445.