Superoxide dismutases (SOD) are a group of metalloenzymes (containing Fe, Mn, or Cu and Zn) that catalyze the disproportionation of superoxide free radical (O2•–) to form hydrogen peroxide and dioxygen as shown below (Eq. 1) [1].
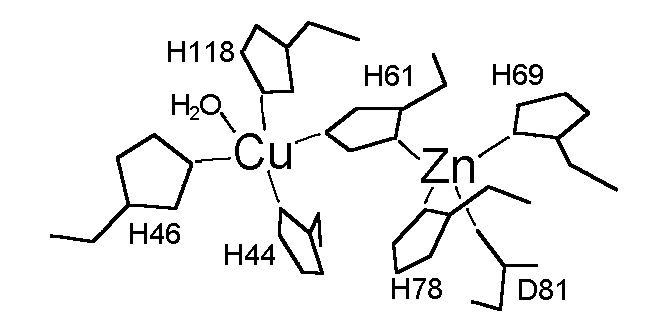
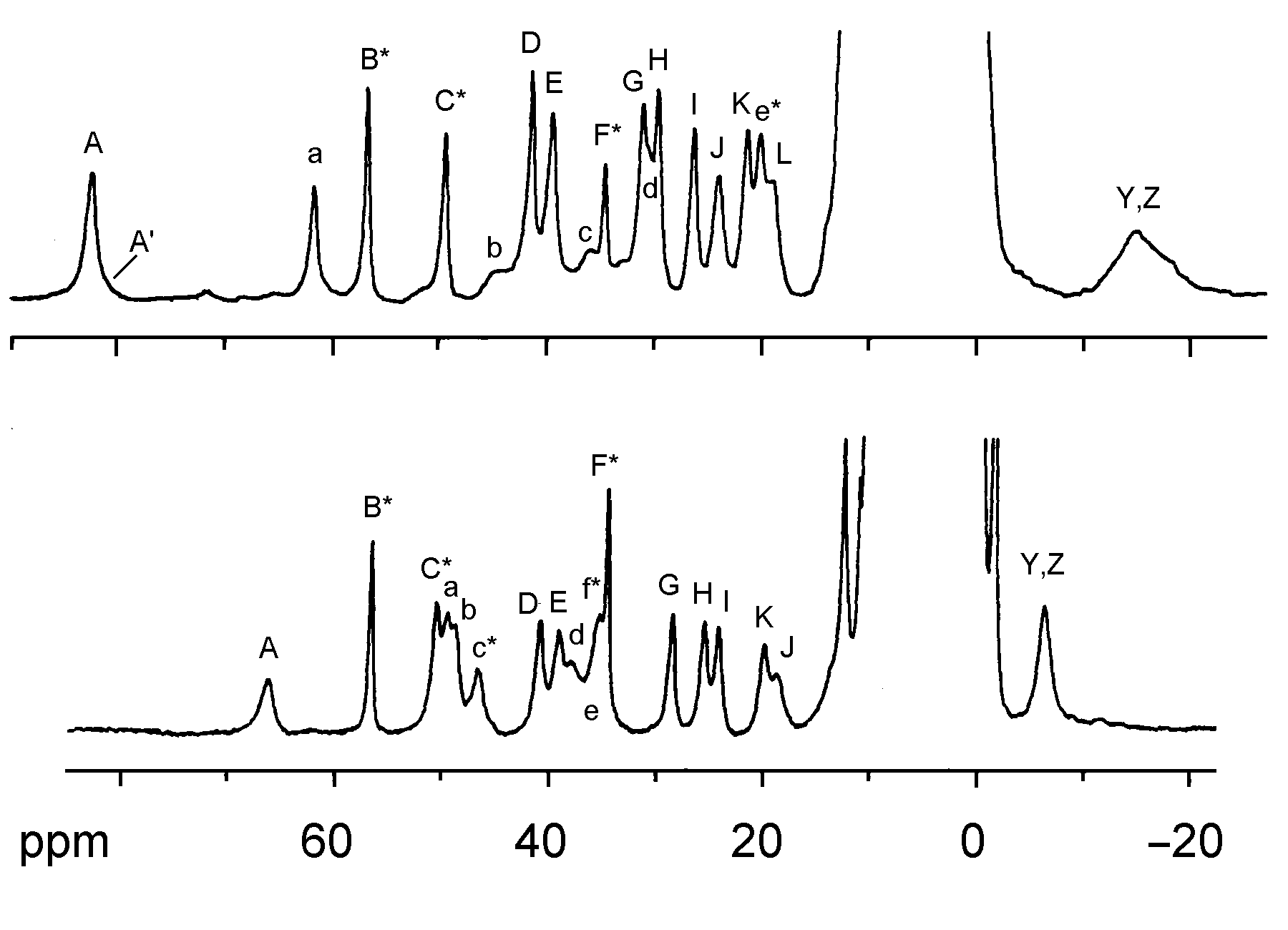
These enzymes have been considered the defense system against the cytotoxic superoxide free radical. Moreover, mutants of Cu,Zn-SOD have recently been found to associate with the Gehrig's disease (familial amytrophic lateral sclerosis) [2]. Cu,Zn-SOD is a prototypical dinuclear metalloprotein that has been subjected to extensive mechanistic and spectroscopic studies. The structure of the enzyme from bovine erythrocytes (dimer with MW = 32,000 Da) has been determined at 2.0 Å resolution by the use of X-ray crystallography which revealed a distorted square pyramidal Cu2+ and a distorted tetrahedral Zn2+ in the active site [3]. The Cu2+ site is responsible of the catalysis and is coordinated by four His residues (His-44, His-46, His-61, and His-118) and a water molecule, and the Zn2+ site is bound to the protein through His-61, His-69, His-78, and Asp-81 (Figure 1). The two metal ions are 6.3 Å away from each other and bridged by the imidazolate of the His-61 residue. The positively charged Arg-141 located in the active site channel about 5 Å away from the Cu has been proposed to play a key role in the catalysis by providing an electric gradient to attract the negatively charged O2•– substrate. Studies focusing on this residue by means of chemical modification [4] and inhibitor (phosphate) binding [5] also indicate the significance of this residue in Cu,Zn-SOD catalysis.

References
[1] Valentine, J. S., and Pantoliano, M. W. 1981,
in Copper Proteins, Spiro, T. G., (Ed.), Wiley: NY, Vol. 3, Chapter 8.
[2] (a) Radunovic, A., Shaw, C. E., Akman-Demir,
G., Idrisoglu, H., and Leigh, P. N. 1997, "CuZnSOD-associated amyotrophic
lateral sclerosis" Ann. Neurol., 42, 273-274. (b) Hart, P.
J., Liu, H., Pellegrini, M., Nersissian, A. M., Gralla, E. B., Valentine,
J. S., and Eisenberg, D. 1998, "Subunit asymmetry in the three-dimensional
structure of a human CuZnSOD mutant found in familial amyotrophic lateral
sclerosis" Protein Sci., 7, 545-555
[3] Tainer, J. A., Getzoff, E. D., Beem, K. M.,
Richardson, J. S., and Richardson, D. C. 1982, "Determination and
analysis of the 2 Å-structure of copper, zinc superoxide dismutase"
J. Mol. Biol., 160, 181-217
[4] Mota de Freitas, D., Ming, L.-J., Ramasamy,
R., and Valentine, J. S. 1990, "35Cl
and 1H NMR Study of Anion Binding to Reduced Bovine Copper-Zinc Superoxide
Dismutase" Inorg. Chem., 29, 3512-3518.
[5] (a) Mota de Freitas, D., and Valentine, J. S.
1984, "Phosphate is an inhibitor of copper-zinc superoxide dismutase"
Biochemistry, 23, 2079-2082. (b) Mota de Freitas, D., Luchinat,
C., Banci, L., Bertini, I., and Valentine, J. S. 1987, "31P
NMR study of the interaction of inorganic phosphate with bovine copper-zinc
superoxide dismutase" Inorg. Chem., 26, 2788-2791.
[6] (a) Ming, L.-J., and Valentine, J. S. 1987,
"Preparation and characterization of Cu2Ni2
and Ag2Ni2
superoxide dismutase, two new metal-substituted derivatives" J. Am.
Chem. Soc., 109, 4426-4428. (b) Ming, L.-J., Banci, L., Luchinat,
C., Bertini, I., and Valentine, J. S. 1988, "Characterization of
copper-nickel and silver-nickel bovine superoxide dismutase by 1H
NMR spectroscopy" Inorg Chem., 27, 4458-4463.
[7] Ming, L.-J., and Valentine, J. S. 1990,
"NMR studies of nickel(II)-substituted derivatives of bovine copper-zinc
superoxide dismutase with nickel(II) bound in the copper site" J.
Am. Chem. Soc., 112, 6374-6383.
[8] Bertini, I., Luchinat, C., Ming, L.-J., Piccioli,
M., Sola, M., and Valentine, J. S. 1992, "Two-dimensional 1H NMR
studies of the paramagnetic metalloenzyme copper-nickel superoxide dismutase"
Inorg. Chem., 31, 4433-4435.
[9] Bertini, I., Lanini, G., Luchinat, C., Messori,
L., Monnanni, R., Scozzafava, A. 1985, "Investigation of Cu2Co2SOD
and its anion derivatives. 1H NMR and electronic
spectra" J. Am. Chem. Soc., 107, 4391-4396.