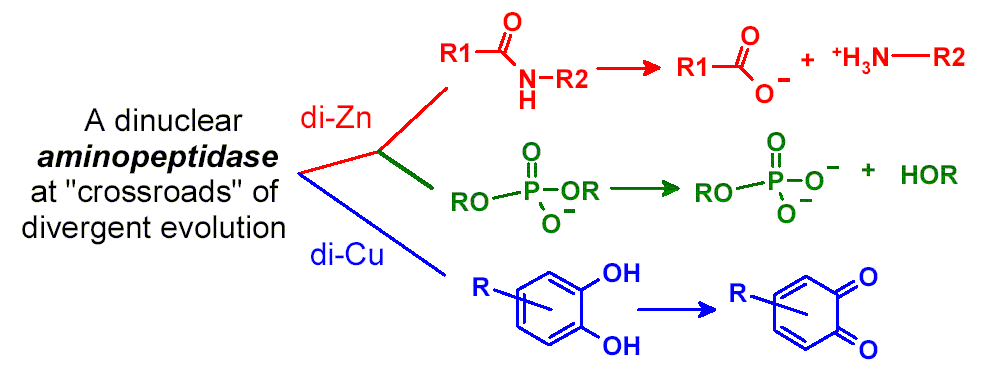
Catechol Oxidase Activity of Di-Cu2+-Substituted
Aminopeptidase from Streptomyces griseus
J. Am. Chem. Soc. 2005, 127, 16380-16381.
Giordano F. Z. da Silva and Li-June Ming*
Department of Chemistry and Institute for
Biomolecular Science, University of South Florida, 4202 E. Fowler Avenue,
Tampa, Florida 33620-5250
Streptomyces griseus aminopeptidase exhibits activities toward the hydrolyses
of peptides and bis(p-nitrophenyl)phosphate (40 billion folds), and catechol
oxidation reported herein with catalytic efficiency kcat/Km
only about 10 times smaller than that of gypsywort catechol oxidase.
The multi-functionality of this enzyme suggests that it is a unique system
for further exploration of protein structure and function and a template
for design of enzymes of diverse activities.
(reprint
in pdf)