Abstract
The phosphatidylcholine-preferring phospholipase C from Bacillus
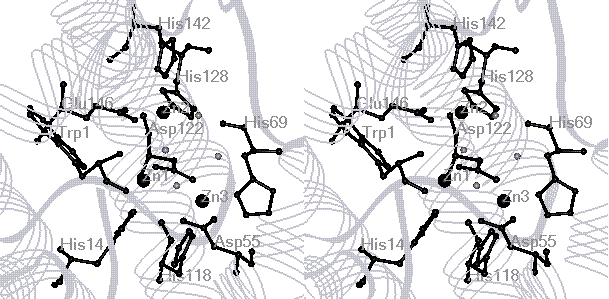
cereus (PC-PLCBc) is a tri-Zn enzyme with two "tight binding"
and one "loose binding" sites. The Zn2+ ions can be replaced
with Co2+ and Cu2+ to afford metal-substituted derivatives.
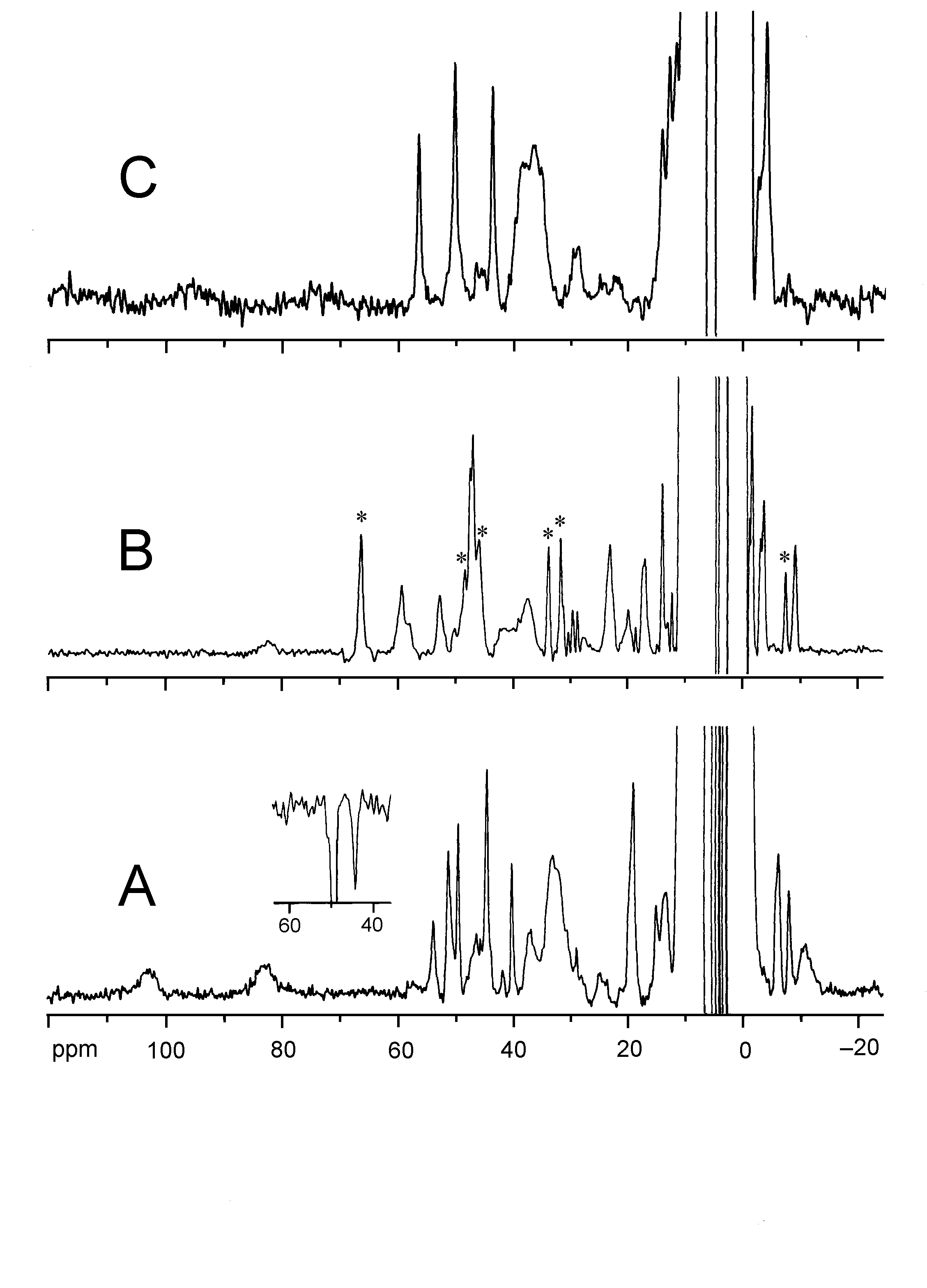
Two Cu2+-substituted derivatives are detected by means of 1H
NMR spectroscopy, a "transient" derivative (Spectrum
A, below) and a "stable" derivative (Spectrum B,
below). The detection of sharp hyperfine-shifted 1H NMR
signals in the "transient" derivative indicates the formation of a magnetically
coupled di-Cu2+ center, which concludes that the Zn2+
ions in the dinuclear (Zn1 and Zn3) sites are more easily replaced by Cu2+
than that in the Zn2 site (See active-site structure). This might possibly
be the case for Co2+ binding. Complete replacement of the 3
Zn2+ ions can be achieved by extensive dialysis of the enzyme
against excess Cu2+ to yield the final "stable" derivative.
This derivative has been determined to have 5 coordinated His residues
and an overall S’ =1/2 spin state with NMR and EPR, consistent with the
formation of a tri-Cu2+ center (i.e., a di-Cu2+/mono-Cu2+
center) in this enzyme. The binding of substrate to the inert tri-Cu2+
center to form an enzyme-substrate (ES) complex is clearly seen in the
1H NMR spectrum (Spectrum C, below),
which is not obtainable in the case of the native enzyme. The change in
the spectral features indicates that the substrate binds directly to the
trinuclear metal center. The studies reported here suggest that 1H
NMR spectroscopy can be a valuable tool for the characterization of di-
and multi-nuclear metalloproteins using the "NMR friendly" magnetically
coupled Cu2+ as a probe.