Comprehensive 2D 1H NMR Studies of Paramagnetic Lanthanide(III) Complexes of Anthracycline Antitumor Antibiotics
Xiangdong Wei and Li-June Ming*
Department of Chemistry and Institute for Biomolecular Science
University of South Florida, Tampa, Florida 33620-5250
Synopsis
Several paramagnetic lanthanide(III) complexes of
the anthracycline antitumor antibiotics, daunomycin and adriamycin (see
structures), have been prepared under several different conditions.
The isotropically shifted 1H NMR spectrum
of a 1:1 Yb3+-daunomycin complex (1)
has been completely assigned, from which its configuration in solution
can be determined. These complexes with different stoichiometries
1:2 (2), 1:3 (3), and 2:1 (4) can be prepared under
different proton and metal concentrations, and have been characterized
by using EXSY technique. These Yb3+-anthracycline
complexes are used as model systems for further understanding of the binding
of anthracycline antibiotics with alkaline earth metal and transition metal
ions. These studies may shed light on the effect of metal ions on
the action of these quinone-containing drugs.

Abstract
The binding of several lanthanide(III) ions to anthracycline
antitumor antibiotics, daunomycin and adriamycin, in methanol and aqueous
solutions has been studied by means of optical and 2D NMR (COSY, TOCSY,
and EXSY) techniques. These results indicate that a 1:1 Yb3+-drug
complex (1) is the predominant complex at a metal to ligand ratio
<10 with slightly higher proton activities, e.g., ~pH 4-5 in an aqueous
solution. In the presence of a base, 1:2 (2) or 1:3
(3) Yb3+-drug complex can be formed.
In addition, a 2:1 complex (4) is formed when the metal-to-drug
ratio is >25. These Yb3+-drug complexes
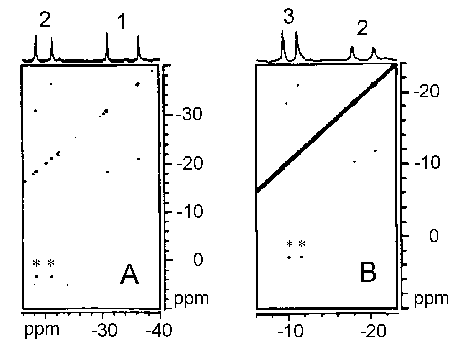
undergo slow chemical exchange with each other relative to the NMR time
scale. Therefore, 1D and 2D magnetization transfer experiments can
be utilized for the assignment of the isotropically shifted signals arising
from the drug nuclei in the various paramagnetic complexes. The spin-lattice
(T1) relaxation times and solution magnetic susceptibilities
of these Yb3+-drug complexes confirmed
the binding of the metal ion to 11,12-a-ketophenolate
in all the complexes (except the second Yb3+
in the 2:1 complex which binds to the 5,6-a-ketophenolate).
Several other lanthanide(III) ions Pr3+,
Eu3+, and Dy3+
show similar binding properties to daunomycin based on optical and NMR
studies. The binding of Yb3+ to daunomycin
has a profound effect on the reduction potential of the drug, showing a
decrease in the potential by 150 mV upon addition of 1 equivalent Yb3+
to the drug solution. This observation indicates that metal ions
must play a significant role in the action of these family of drugs in
vivo.
(Read an introduction about
anthracyclines)
(Back to Current Publications)